Laure PILLIER*, Florent KRAVTCHENKO, Mirna SHAMAS, Sébastien BATUT, Bénédicte CALIMET, Christa FITTSCHEN
PC2A - Physico-Chimie des Processus de Combustion et de l'Atmosphère - UMR 8522
Context and objectives
This research project concerns atmospheric chemistry, and more particularly the study of chemical reactions involving radical species in the atmosphere.
Hydroxy OH, hydroperoxy HO2 and peroxy RO2 radicals play a key role in atmospheric chemistry through the radical cycle presented in Figure 1, that involves nitrogen oxides (NO and NO2) and controls the oxidative capacity of the atmosphere, the formation of tropospheric ozone and secondary pollutants
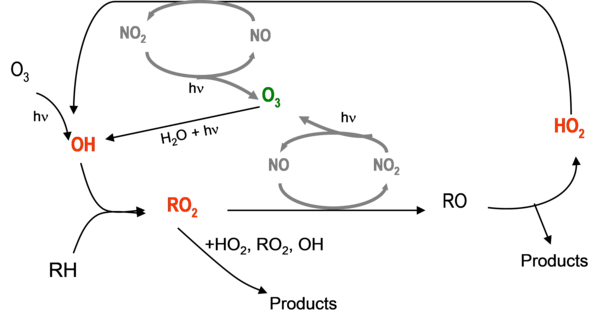
OH radical is the main oxidant in the troposphere (diurnal) and it will oxidize essentially every organic species emitted (Volatile Organic Compounds VOCs from biogenic and anthropogenic sources) through the reaction:
RH + OH → R + H2O
The alkyl radical R. (RH is a saturated hydrocarbon) will react with oxygen to form peroxy radicals RO2:
R + O2 + M → RO2 + M
The reaction of RO2 with NO will dominate in regions influenced by anthropogenic NOx emissions, leading to the formation of alkoxy racidals RO along with NO2 which will be photolyzed to generate ozone O3.
In pristine regions of the atmosphere (i.e., in the marine boundary layer or in remote tropical forests), where NOx concentrations are low, reactions of RO2 with HO2 and with other RO2 play a larger role and contribute to the radical removal/chain termination process, forming hydroperoxide ROOH:
RO2 + HO2 → ROOH + O2
Because organic hydroperoxides are relatively stable in the atmosphere, this process is considered as a radical sink, reducing the oxidative capacity of the troposphere.
However, recent experimental and theoretical studies have shown that radical termination (via reaction with HO2) may not be the exclusive reaction pathway for certain RO2. It can lead to three different reaction pathways:
RO2 + HO2 → ROOH + O2 (a)
→ ROH + O3 (b)
→ RO + OH + O2 (c)
In this case, it is primordial to know the branching ratios of the different pathways for a better knowledge of the oxidation mechanism of VOCs present in the atmosphere.
Moreover, very recently, a new reaction pathway has been suggested as possible fate of RO2 radicals in clean environments: the reaction of RO2 with OH radicals. Only few studies exist on this class of reactions and to date, they are not included in atmospheric chemistry models.
Reactions between RO2, HO2 and OH radicals are (or not) included in the chemical mechanisms of atmospheric models and the uncertainties and/or lack of data on reaction rates and branching ratios can lead to important errors on modelling of ozone and radicals concentrations in the atmosphere. New experimental results, with a more detailed characterisation of radicals and products, are then clearly needed.
The aim of this project is the development of a new experimental device to study reactions between RO2, HO2 and OH, and to measure the rate constants and branching ratios. The new setup consists of a fast flow tube (several m.s-1) coupled to two laser diagnostics: Laser Induced Fluorescence (LIF) for OH radicals measurements, continuous wave-Cavity Ring Down Spectroscopy (cw-CRDS) for HO2 radicals measurements; and Mass Spectrometry analysis associated with Molecular Beam (MB/MS) sampling for stable species measurements. The coupling between those three techniques with a fast flow reactor is done, as far as we know, for the first time.
This scientific project is part of the Labex CAPPA (Chemical and physical Properties of the Atmosphere, 2012-2019 WP1 : From gas phase to aerosols: biogenic volatile organic compounds (BVOCs) as precursors for particles) and the CPER project Climibio ( changement climatique, dynamique de l'atmosphère et impacts sur la biodiversité et la santé humaine, 2014-2020, WP1 : Méthodes d'évaluation de la qualité et du changements des milieux).
Experimental setup
The fast flow reactor technique is known to be well adapted for kinetic studies of elementary reactions, and particularly radical + radical reactions. Rate constants are generally measurable in the range 10-14 - 10-10 cm3.molecule-1.s-1 in a temperature range usually varying from 200 to 1000 K. This technique is used at low pressure (<1.33 kPa (10 torr), for viscous and laminar flow conditions). The determination of kinetic parameters is usually done using the pseudo-first order approximation where one of the reactant is in excess compared to the other one, its concentration is therefore considered as constant during the reaction.
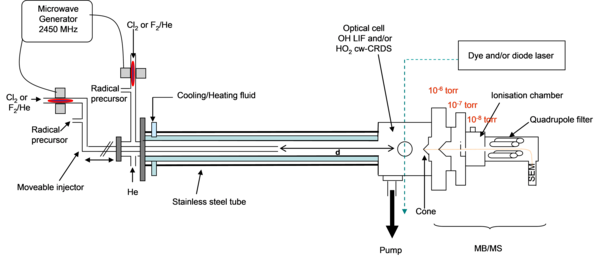
Our reactor (Figure 2) includes a fixed stainless steel tube (75 cm length and 2.5 cm inner diameter) and an injector (in Pyrex, 83 cm length and 0.8 cm i.d.) which can be moved relative to the main tube allowing the variation of the reaction time between the two reactants. The flow in the reactor is viscous and laminar, comparable to a plug flow reactor, then the reaction time t can be defined by the relation:
t = d/V
where d is the distance between the injector and the end of the fixed tube and V the mean flow velocity in the reactor.
Radicals are generated by microwave discharge (2450 MHz – 200W max, Sairem GMS200W) in both tubes, which are coated with HalocarbonÒ wax to minimize the heterogeneous loss of radicals on the walls. The pressure in the reactor is maintained between 0 and 1.33 kPa (10 torr) using a rotary vane pump (UNO 120, Pfeiffer) and controlled by a BaratronÒ pressure gauge. Reactants are highly diluted in Helium.
To control the temperature in the reactor, it is enclosed in a double wall cylinder with a cooling or heating circulation system. The end of the reactor is provided with an optical cell with five optical accesses for LIF and cw-CRDS measurements and a sampling cone for MB/MS measurements.
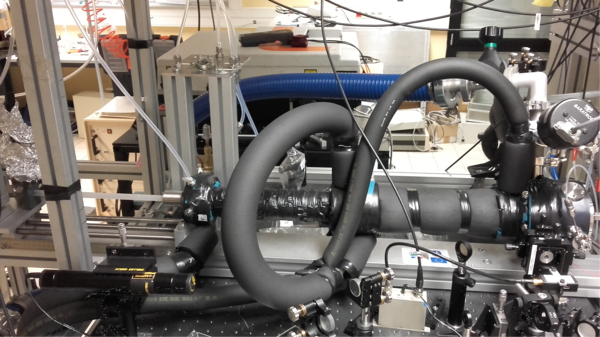
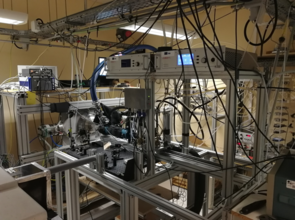
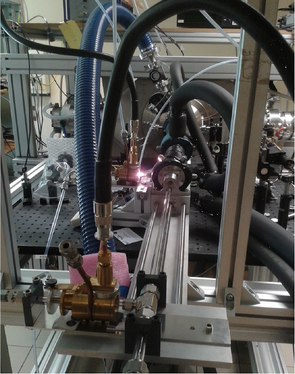
Figure 2: Schematic view and pictures of the reactor